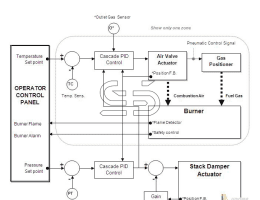
What is steel? Iron-carbon alloys with minor amounts of some other elements such as manganese, silicon and … (Steel) Their carbon content can range from 0.025 to 2. This group of materials, due to having properties Proper metallurgy has many applications in the industry. Pure iron is very soft and does not have much industrial use and the addition of alloying elements to iron can cause Improves its mechanical properties. Among the elements, carbon is the most important element on the mechanical properties of iron It has a positive effect and in many cases determines the mechanical properties of iron alloys. Steels are iron-carbon alloys with carbon content ranging from 0.025 to 2% and In addition to carbon they contain a percentage of some other alloying elements. Steel is the most widely used industrial metal and its consumption is steadily increasing. Today the world’s steel production is close to a thousand And that’s three hundred million tons a year. It is said that steelmaking came from India to Iran because of the era Ancient, Indian steel has been called. Steel is multi-welded. To make steel, use white carbon steel ش Lose weight, measure other elements in it to make the steel burnt from 0.5 to 2.5 to 1.5 to 1/2 Required to be made. Alloy steels are often associated with other metals. Properties of steel to the percentage of carbon contained in it, heat treatment It depends on it and the alloying metals in it. Introduction to all kinds of steel In most engineering applications, steel is a major contributor. It is heavy and capable of transmitting forces. Steel Classification Methods: By chemical composition they are divided into the following groups: A (Carbon Steel Plain: There are steels that are the main constituent of carbon. 2 Carbon steel is divided into three groups:
- Low Carbon Steel) Max. Carbon (
- Low Carbon Steel) Max. Carbon (
- Carbon steel) over 55 /. Carbon ( B (Low Alloy Steel: There are steels with a maximum content of 5% of alloying elements. C (alloy steel: Alloys in these steels account for more than 5%. Percentage of carbon in low-alloy and high-alloy steels. is . According to the application and methods of heat treatment of steel, the following types are divided: Cementation steels – Heat treatment – Nitrogen – Bearing – Stainless steel … Group 1: Cementation steels The carbon content of these steels is low and a maximum of 0.3.These steels are in addition to carbon alloys such as manganese, They contain silicon, crom, molybdenum and nickel and are produced under heat-treated carbon. Due to Low carbon content after hard heat treatment will not be high hardness. Carbonation becomes high-carbon hardness rises to the surface. The maximum carbon penetration will be 2 mm below the surface. Steels Hard and soft surface cementitious, soft and tough and with high abrasion resistance, while resisting They also have a high impact. Group II: heat-treated steels The carbon content of these steels is more than / 3. So, as a result of heat treatment in oil or water, they are hardened . To improve the mechanical properties of these steels, some alloying elements are added. These steels Harden to the brain), especially parts that are thin and cool in water. 3 After heat treatment they should be immediately sealed to prevent possible cracking. The alloy and carbon are divided into two. Simple carbon steels are steels that have small amounts of elements Alloys such as manganese) are up to 9 /.( maximum) heat-treated brain parts made of this steel. Not so hard. The general reasons for adding alloying elements to steels are: 1- Increased hardness 2- Increase the strength of the piece at high and low temperatures 3. Increased wear resistance 4- Increase magnetic properties 5. Increase the energy needed to break the piece Group Three: Spring steels When a piece is subjected to external forces it shows itself before breaking two kinds of deformation. Elastic deformation (elastic) Under these conditions, if the force is removed from the body, the body returns to its original state. 2- Plastic deformation of castors Under these conditions, if force is removed from the body, the body will not return to its original dimensions. Which, as a result of the application of force and after its removal, return to their original form and dimensions. Because of being high The forces and deformations that are applied to the springs used in the industry are required to meet the elastic limit of the piece. Elevation means that steel under high stresses of 21000 (kg / mm2) can exhibit elastic deformation from The springs are subject to varying forces and therefore the steel required for these components must be of fatigue resistance. As a result, springs are used for making springs having good amounts of Silica, Mn, Cr, Vanadium and Molybdenum. Heat-resistant steels contain Cr, Vanadium And tungsten. Corrosion-resistant steels contain cream, nickel and molybdenum (stainless steels). 4 Group IV: Stainless steel The amount of sulfur in the steel should be low because the steel during forging, forging (and hot rolling) exhibits brittle behavior. Shows and the workpiece cracks even though steel machining capability is needed And instead of long cuts, short cuts are added to steel to a certain extent (35 -. / 08 /. . To increase the machinability of steel, lead and phosphorus-containing steels can be combined with sulfur (2 – 15/15). used . Lattice steels are not suitable for hot-rolled forging and rolling operations. Phosphorus Also if its value is more than 2 /. The impact strength of the steel is reduced The use of stainless steel may be for the following reasons: 1- Long tool life due to low friction 2. High cutting speeds 3 – Low power requirements 4- High precision in machined parts 5. Smooth surface after machining prevents subsequent operation 6- Minimum dressing tool due to very low wear. Fifth group: Bearings and bearings Bearings and bearings are parts that are subject to extreme wear and compression forces, so Steels that have the high abrasion resistance and do not get crushed by compressive forces. Suitable for this purpose should contain high carbon (about one percent (and relatively high cream) about 1.5 percent). Which will increase their hardness to 64 HRC after heat treatment? Group 6: Nitration steels Nitrogen (N) is introduced into the steel surface by heat treatment of nitration on steels. Operation in a nitrogen-containing environment at 580 – 480 ° C on special steels below 5 They will be referenced to be done. To perform this operation the parts are first heat-treated, tempered and if applicable They also need machining. Also, after the operation there is no need for high-temperature parts in cooling environments It can be cold like water or oil so the risk of cracking is also low. Other benefits it can be The crystalline structure is inexpensive and unaltered. There are more cementation and less penetration. Aluminum-containing steels are the most important type of nitrated steels